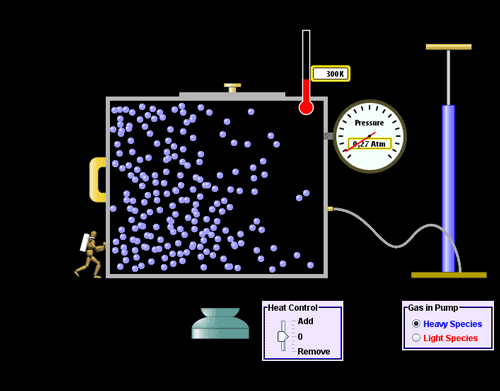
The
invisible properties of gas
|
- Gas assumes the shape
and
volume of its container particles
can move past one another.
- Gas is compressible
because
there is lots of free space
between particles.
- Gas flows easily
because particles
can move past one another.
____________________________
|